- Talkdog
- Tourmaline
- Facial steamer spa
- Quantum Magnetic Resonance Analyzer
- Detector and therapeutic apparatus
- Fir Infrarred Massage House
- Laser Comb
- Facial Beauty Wand
- Biocell charger
- Water Ionizer
- Ion Cleanse
- Ion Array
- Massage Bed
- Chi Machine
- Detox Foot Patch
- FIR Foot Sauna
- Basalt Massage Stone
- FIR Massage Mattress
- Oxygen Concentrator
- Beauty Equipment
- Weight Losing Machine
- Massage Instruments
- Hydropathic Spa
- Massage Bed Pad
- Nail Printer
- magnetotherapy device
- Other Product
Home>>QA Department
QA Department
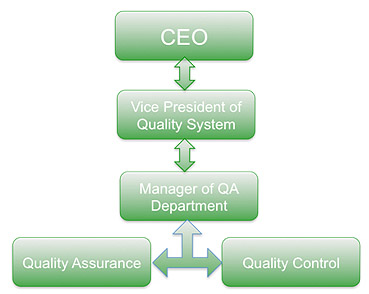
QA Department
Quality Assurance Department provides Qualitative Support to all the departments of the company as to ensure customer satisfaction. There are two basica functions integrated in QA department.
Quality Assurancecovers all activities from design, development, installation, production, transportation, shipment and documentation. This introduced the rules: "fit for purpose" and "do it right the first time". It includes the regulation of the quality of raw materials, assemblies, products and components; services related to production; and management, production, and inspection processes. Quality Assurance functions as the PDCA (Plan-Do-Check-Act) approach, also known as the Shewhart cycle.
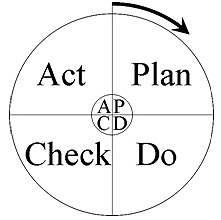
Quality Control - operational techniques and activities intending to ensure that a manufactured product or performed service adheres to a defined set of quality criteria or meets the requirements of quality standards.
QA Department has broad responsibilities and authority in the following areas:
1.Quality Improvement - Quality improvement is based on the premise that all work activities can be planned, performed, measured, and improved. QA monitors our progress toward our corporate goal of building a culture in which improvement is continuous and an integral part of the organization. QA department is making efforts to improve quality system continuously to provide the highest quality products and services to domestic and international customers.
2.Personnel GMP Training and Qualification - all employees who come into contact with our products must begin GMP training within the first month of employment, GMP training continues on a regular basis throughout the length of employment. Tests are given to monitor the effectiveness of training.
3.Internal Audits - QA inspectors monitor all phases of production to assess performance and adherence to GMP and to the SOPs of each department.
4.External Audits - QA oversees and supervises inspections and audits of our facilities by domestic and international regulatory bodies, as well as by customers and independent auditing firms.
5.Supplier Qualification - QA department maintains an audit program to verify our suppliers' ability to provide consistent products that meet our strict quality requirements.
6.Document and Record Control - QA is responsible for maintaining all documents, records and Standard Operating Procedures, making sure that they are up to date.
7.Inspection and Acceptance Testing - QA has the authority to release and reject any component or finished product that does not meet specifications.
8.Non-Conformances - QA handles the identification, documentation, control, investigation and disposition of all non-conforming materials, components and final products.

